Monarch Disease Control
Jacob Groth
The following information was put together for a presentation on this topic at the 2000 IBBA convention in Kansas City, MO. It was supported with a presentation of about 25 pictures. But unfortunately, the majority of those pictures were not scanned, so I am unable to attach them to this paper. I will, however, attach pictures of the Oe spore (here).
The outline of this report is as follows:
Monarch Disease Control
1. Bacteria
- Microscope magnification needed
- Symptoms
- Treatment
2. Malnutrition / toxic poisoning
- Symptoms
- Treatment
3. Ophryocsystis elektroscirrha (Oe)
- Microscope magnification needed
- Symptoms
- Treatment
- Microscope magnification needed
- Symptoms
- Treatment
Please keep in mind that this is a very basic outline. It’s not a comprehensive study or report on these issues. But nevertheless, I hope you will find it to be helpful.
1. Bacteria
Microscope magnification recommendation: 400x
- It is hard to identify bacteria beyond a generic level as there are many different types. But, for our purposes, it is not all that necessary to identify the specific types (bacilli, cocci, etc.). Fortunately, it is fairly easy to recognize generic bacteria as they move quite rapidly under the microscope. Most healthy caterpillar cells do not move rapidly like a bacteria organism.
Symptoms:
- Foul odor (sometimes like bad breath) in rearing container.
- Caterpillar stops eating and moving, usually dies shortly thereafter.
- Caterpillar turns “mushy.”
- Caterpillar fluid is brown, black, or purple.
- Smelly or runny reddish-purple frass.
- If pupates, chrysalis turns mushy, black, or purple with a foul odor upon breaking open.
Caution: although it is fairly easy to identify and remedy bacteria in general, the bacteria may simply be a secondary infection due to a larger problem. For example, if a caterpillar is infected with a polyhedrosis virus, it will also eventually become infected with bacteria. When looking under the microscope at a dissected caterpillar gut, it is likely that there will be a lot of bacteria dominating the slide, even thought the primary cause was the virus.
Treatment:
- I have heard that antibiotics have been successfully used for treatment of bacterial infections. Personally, I do not recommend this. First of all, it would be necessary then to identify which bacteria the caterpillar is infected with. Second, antibiotics are only a temporary treatment, as the bacteria will eventually build up a tolerance to the antibiotics. Finally, antibiotics do not resolve the original problem of how the bacteria got there in the first place.
- I believe the best approach is preventative measures. Bacteria is usually caused by poor rearing methods; usually a combination of heat, humidity, lack of airflow, overpopulation, and lack of sterilization. The most important of these is airflow. The airflow will naturally keep the humidity and temperatures (if rearing in lab conditions) at a proper level.
- The caterpillars should be kept in a clean and sterile environment and kept from exposure to their own frass, as this is primarily the breeding ground for the bacteria.
- Containers should be sterilized daily and fresh milkweed replaced one to two times per day.
- Finally, please be sure to look at the final suggestions and conclusions about rearing methods.
2. Malnutrition and / or toxic poisoning (pesticides, etc.)
Symptoms shown by the caterpillars
- High casualty rate during 1st and 2nd instar.
- Caterpillars may tend to walk of the plants in search for better food.
- 3rd – 5th instar caterpillars may be found lying on the bottom of your container or pot in a semi-curled position (not their defensive fully curled position). Usually, they will die shortly. Sometimes they will be wiggling on the ground and expelling green fluid out of both ends.
- The caterpillar may not be able to fully pupate, can only push halfway through it’s skin.
- The chrysalis may be slightly deformed or have a bumpy texture rather than it’s normal smooth shell.
Treatment
- The first three points above are likely caused by pesticides, bleach residue, or some form of toxic poising. The best thing to do is to fully rinse your plants to ensure all residues are removed. If this does not work and symptoms prevail, it may be possible that the plant has a systemic pesticide. At this point, it would be necessary to change your milkweed source.
- The second two points are likely caused by malnutrition. I would recommend trying to get a hold of a fresher source of milkweed. Usually, the above final two symptoms are found when feeding late season wild milkweed that is somewhat dried and lacking in nutrients. It may not be possible to try a new milkweed source, but it is highly recommended as this is likely your problem.
3. Ophryocystis elektroscirrha (Oe)
Microscope magnification recommendation: 100x - 400x
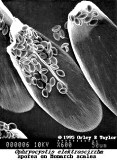
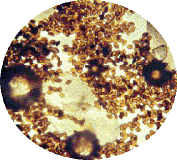
- Oe is relatively easy to spot under the microscope. The best method is to take a scale sample from the abdomen of the adult butterflies. The spores primarily appear on surface of the abdomen close to the reproductive organs. Simply, take a strip of double-sided tape and make a “finger print” impression from the bottom end of the abdomen on the butterfly to be tested. The more scales that are removed, the better the test. Then, place the tape on a microscope slide and look for the protozoa spores. They very much resemble the shape of a football, see attached pictures (spores magnified about 250x).
Symptoms
- Not as noticeable in the caterpillar, but in worst cases, the caterpillar may die at 5th instar or be unable to pupate.
- First sign could be a dark edge along the wing tips of the chrysalis.
- Later pupa stage (in serious case) will show a brown abdomen, especially the day before emergence.
- Chrysalis shell, especially around the abdomen, will be less transparent or even “dirty” looking.
- Lighter case: the butterfly will emerge fine, but have a “dirty” looking abdomen. The butterfly should visually have a very clear and crisp black and white scale pattern. This butterfly will likely only live a few days. They usually die from dehydration as the protozoan appears to affect its ability to retain moisture.
- Lighter case: the abdomen will appear green due to a lack of scales and a thin skin lining. The green color is not the protozoan. It is simply that due to the effects of the thin skin lining and lack of scales caused by infection of the protozoan, the inside of the butterfly’s abdomen is showing through.
- Heavier case: the butterfly will emerge from its chrysalis but have wrinkled or deformed wings. This butterfly will not likely live beyond 24 hours.
- Heaviest case: the butterfly will be unable to emerge. It will either be “stuck” by its abdomen in the chrysalis, struggling to emerge, or it simply won’t emerge at all. In either case, these butterflies will not live.
Special considerations
- The cause of infection is through ingestion by the caterpillar. The protozoa multiplies in the caterpillar and the chrysalis, so the earlier the exposure, the greater the infection. For example, a first instar caterpillar may ingest one or two spores and be a highly infected adult, if it makes it that far. Whereas, a 5th instar caterpillar may ingest hundreds of spores the day before it pupates and end up less infected than the smaller caterpillar.
Treatment
· With the above special consideration, it is clear that early prevention is extremely important. Although prevention and sterilization throughout the whole process is important, it is exponentially so at the earlier stages.
- Start with inspected and selected breeding stock that is Oe free.
- Sterilize all plant material using 10% bleach solution and rinse at least 3 times. This should be done, not only for breeding materials, but all of the feeding material used up until pupation.
- Sterilize eggs if possible using a surface decontamination method. There are egg sterilization treatments available to treat for Oe. This is a secondary measure in case the selection of clean breeders was not properly accomplished.
- Keep all caterpillars separate and isolated from adult butterflies – create “fire-walls.”
- Sterilize rearing tools, containers, etc. using at least a 10% bleach solution on a daily basis. Due to our large volume, we have a wash station where all our sterilization is done and we have one or two employees dedicated to doing all the washing all day long.
Final comments on Oe
- If spores are detected above an acceptable level (greater than 10 spores counted using “finger print” method), the butterfly should not be released into the environment. For breeding purposes, there should be no spores detected. If less than 10 are detected, do not use for breeding, but it will still be a healthy butterfly that can be released into the environment (see Effects of the Protozoan Parasite O. elek. on the Fitness of Monarch Butterflies, by Sonia Altizer and Karen Oberhauser published in the Journal of Insect Pathology). The parasite is commonly found on wild caught monarchs and does not appear to be much of a threat except at the highest dose. But, the real threat of this parasite comes with breeding in large quantities. Ophryocystis elektroscirrha is probably the most common and plaguing disease for monarch breeders.
- The protozoan will naturally be destroyed by a deep freeze. This could be an explanation as to why states in the north have a lower level of Oe found on wild monarchs, where as southern states that do not experience freezes have much higher levels.
4. Polyhedrosis virus
Microscope magnification recommendation: 400x
- Microscope examination should reveal “crystals” or “polyhedra” in the dissected gut of the caterpillar. Crystals are normally present in the insect – primarily uric acid crystals – so don’t be alarmed if you have healthy stock and still find some crystals. In the treatment section, I will discuss how to distinguish between polyhedra and uric acid crystals. Please keep in mind that the polyhedra are very difficult to spot. Usually, there will be a dominant secondary infection if you have a polyhedrosis virus, and so much of what you will see is bacteria. But, do not assume that the bacteria is the primary cause – keep searching for those crystals!
Symptoms
- At or after 3rd instar, the caterpillar becomes sluggish, non-responsive, and simply stops eating, but doesn’t necessarily die right away. A secondary bacteria infection usually shows up a few days later.
- Upon dissection, the caterpillar gut will appear “milky” or “cloudy.” It should appear clear, thus allowing you to see the green color of the milkweed being digested by the healthy caterpillar.
- If it makes it to pupation, the chrysalis is usually small and the butterflies will be extremely weak. If the butterfly emerges, it usually has a hard time hanging on to the pupal shell and will fall to the ground.
- Once these symptoms are noticed, even if only a few cases show these signs, it will be a matter of less than a week before your whole stock will be contaminated. All it takes is one single virus particle to infect the whole stock. It is highly contagious and very difficult to eliminate
Special considerations
- According to insect pathologist Dr. Harry Kaya of UC Davis, cytoplasmic polyhedrosis has never been detected in the wild. It is primarily a laboratory phenomenon. But, obviously, it does exist in the wild, but just at a benign level. It is the laboratory conditions that give the virus a prime opportunity to thrive. So, there is no need to be extremely concerned about exposing your lab-reared stock to polyhedrosis from wild-caught monarchs (it is more likely that the wild-caught monarch will have Oe). But, be extremely careful if polyhedrosis has ever infected your stock in the past. Even if you fully purge your lab and sterilize your equipment, the virus may still be in the soil, on the sidewalks of your property, on the doorknobs in your house, or any other place that you may have been in contact with. It is generally believed that the virus is not air born, but spread through contact. This RNA virus is highly infectious to many different species of lepidoptera (but not all species).
Treatment
- Deep freezes do not kill the virus. UV has shown to kill it, but it is not recommended as a treatment due to all the hazardous side effects accompanied with UV.
- The best treatment is PREVENTION! Please see the final recommendations to learn more about prevention.
- Once the symptoms of polyhedrosis are spotted, it would be wise to examine further for the virus. First, dissect the caterpillar and remove the gut. It should appear clear, thus allowing you to see the green color of the milkweed being digested. If it is “milky” or “cloudy,” the caterpillar likely has an advanced case of cytoplasmic polyhedrosis (or possibly a form of Nosema or some other microsporidium).
- If the gut is “cloudy,” the next step is to check for polyhedrosis. There are more sophisticated ways of detecting the virus, but the most practical way is under the microscope. As mentioned above, at 400x (40 x 10), look for the polyhedra shaped objects in the gut lining. Look long and hard as they are not easy to spot.
- Once the crystal is spotted, the next step is to distinguish between polyhedrosis virus and uric acid. These crystals (polyhedra vs. uric acid) can be separated from each other by the use of dilute NaOH (0.5 Normal sodium hydroxide). Using a small dropper, add a few drops of the solution while observing the crystals under the microscope. The polyhedra will dissolve when the NaOH solution reaches them – whereas the uric acid will not.
- If it is determined that your stock is infected with the polyhedrosis virus, you have a very major overhauling project ahead of you. First, you will need to discard all rearing materials and containers if they cannot be completely sterilized. Anything porous must be destroyed. Anything made of vinyl, steel, plastic, etc. that can be completely immersed can be sterilized.
- It may be necessary to relocate your rearing facility if your rearing was not done in a proper lab and a virus outbreak occurs (proper is defined as a room that can be fully sterilized). If it can be fully sterilized, then do so immediately. If it cannot, then you will need to move to another premises. If you move, I would recommend building a new facility that could be fully sterilized. For example, we have buildings that have vinyl walls, floors, and ceilings. We had to learn the hard way.
- This means that you will also need to destroy all of your livestock, even if they appear to be healthy. This is where many breeders find a stumbling block. If one caterpillar is positively identified with polyhedrosis, it must be assumed that the whole stock is infected.
5. Final recommendations and conclusions
- General practice using sterile methods and procedures is of the utmost importance. Mass production is important for the high volume breeder, but cutting corners in this area will prove to be fatal. There needs to be a fine balance between creating “firewalls” within your stock, while not making it so labor intensive that it is simply not worth the hassle.
- Operate like a hospital. Think sterilization in everything you do. Consider all the things that you come into contact with. Our lab employees are required to where rubber gloves and a lab coat. The labs are cleaned daily. The materials and rearing containers (or anything that comes into contact with the caterpillars) are sterilized daily. There is a difference between clean and sterile. Something that appears clean, may not be sterile.
- Sterilize all food material and rinse thoroughly, at least 3 times. A 10% bleach solution is adequate.
- Most of the discussed diseases are primarily spread through contact. Think in terms of microbiology. For example, if you just handled some butterflies after packaging and shipping and then you walk into your house, you may have just spread the disease to your door-knob – if one of the adults was infected. Then, you wash your hands, close the door behind you (picking up the virus or protozoa that you applied previously), and walk into your breeding lab and spread it to one of your breeders – assuming that your hands were sterile because you washed them. This is the type of thinking that needs to be done when designing your rearing protocol.
- Create firewalls. Always assume that your stock will sometime or another come into contact with any of the above diseases. Separate your stock into small batches. When working between batches, make sure that proper washing procedures take place before working with another batch so as to prevent spread of disease. Separate every stage of your livestock.
- Always assume that your flight cage contains infected livestock – even if it doesn’t. Make sure there is no movement between rearing labs and the flight cage. Make sure there is no contact between adult butterflies and caterpillars.
Jacob has supplied the following photographs to accompany his paper.
Click photo to see enlargement.